RESEARCH HIGHLIGHTS
Below are research highlights from the Pilon lab. Enjoy.
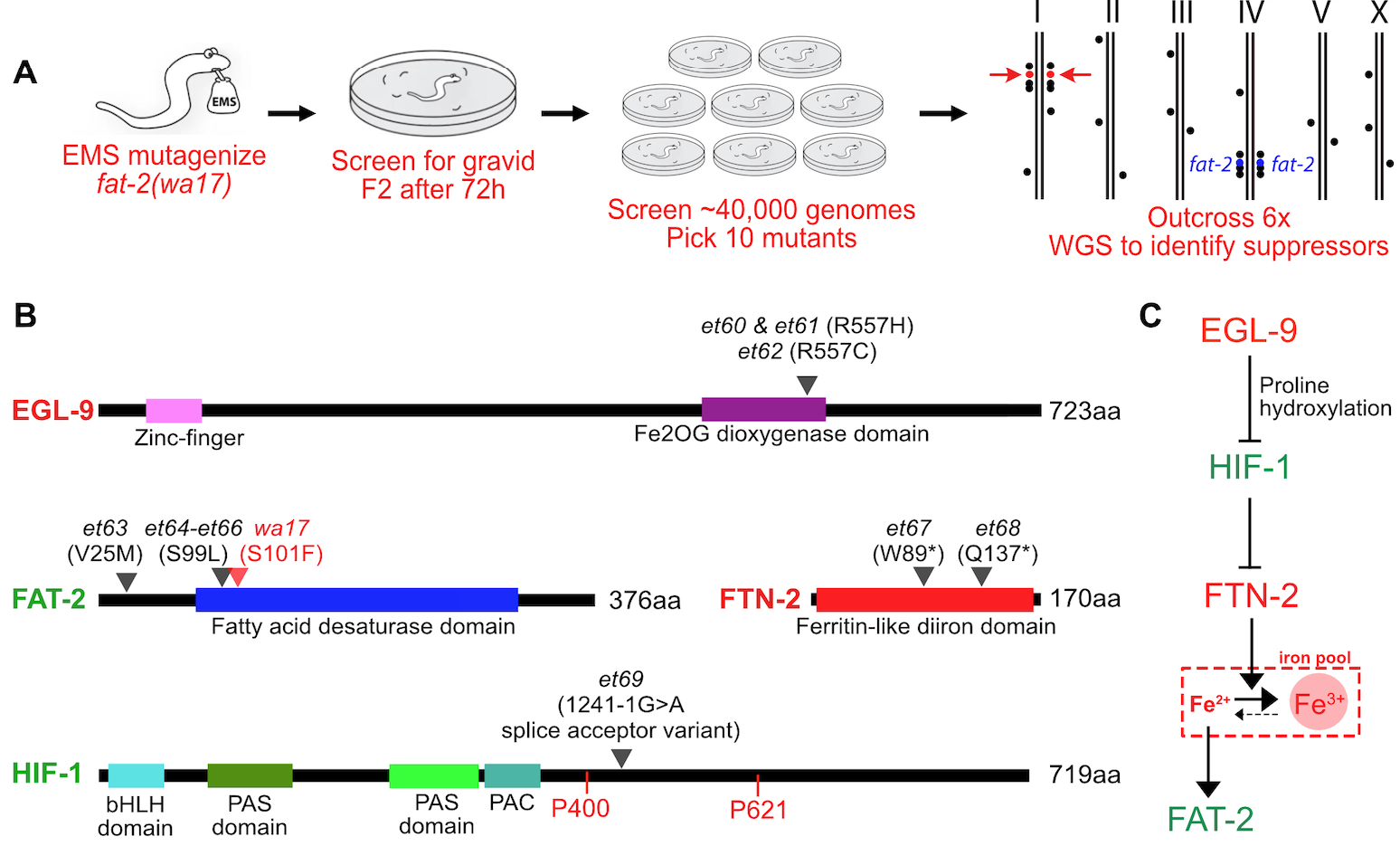
2025: A connection between polyunsaturated fats and hypoxia?
A screen for suppressors of PUFA deficiency identifies alleles within the HIF-1 pathway
We identified novel mutations that compensate for the deficiency in polyunsaturated fatty acids (PUFAs) in the C. elegans mutant fat-2(wa17). In this way, we identified several alleles of genes that encode components of the HIF-1 pathway. These fat-2(wa17) suppressors seem to act by liberating ferrous ions that boost the residual activity of the mutant desaturase encoded by the fat-2(wa17) allele.
Kaper et al (2025) eLife 13:RP104181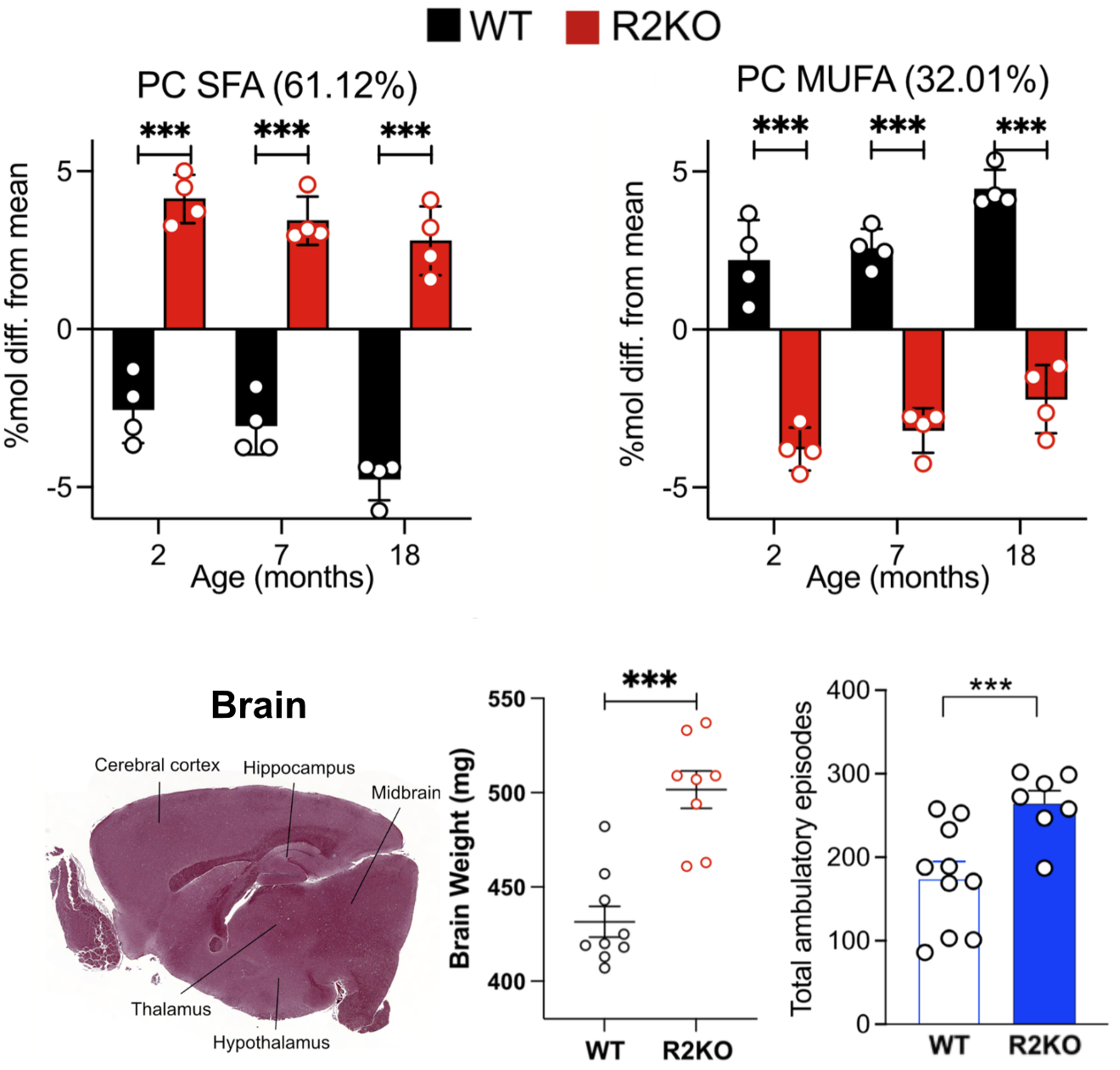
2024: Saturated fat in brain is associated with hyperactivity
We studied behavior and brain composition in AdipoR2 KO mice.
AdipoR2 KO mice have enlarged brains in which the cellular membranes are excessively rich in saturated fatty acids and depleted of monounsaturated fatty acids. These brain defects are accompanied with changes in behavior: the AdipoR KO mice are hyperactive, which may explain why they do not gain weight in old age as the control mice do. Surprisingly, the AdipoiR2 KO mice have a normal life span under standard laboratory conditions.
Ruiz et al (2024) FASEB J. 38:e23815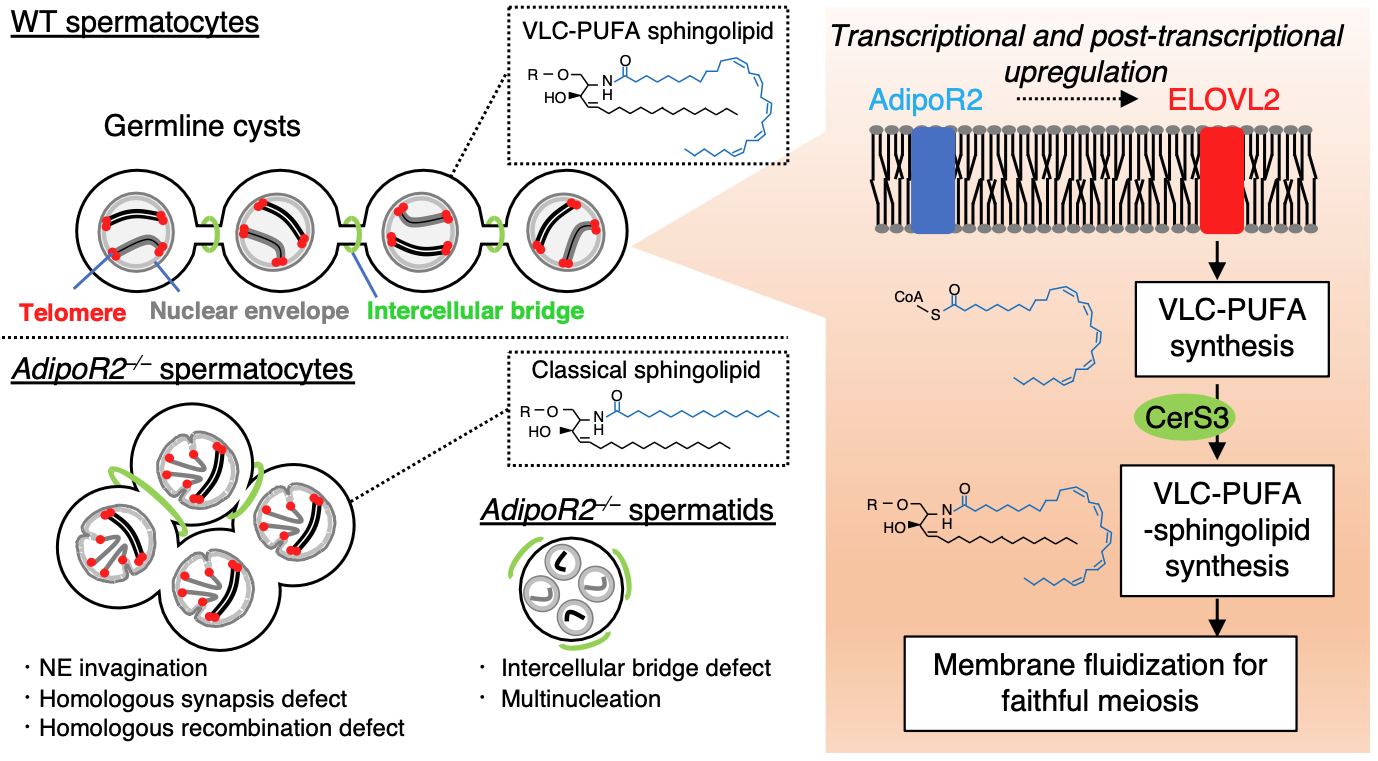
2024: AdipoR2 is essential for spermatogenesis
With the lab of Dr Shibuya, we found that AdipoR2 is required for the production of very long polyunsaturated fatty acids in testis.
We found that several aspects of spermatogenesis are abnormal in AdipoR2 KO mice, including defects in nuclear envelope, defects in homologous recombination and defects in the intracellular bridges that connect developing spermatocytes. The mutant mice lack critical very long chain polyunsaturated fatty acids and the membranes of developing spermatocytes are measurebly rigid and morphologically abnormal. The AdipoR2 KO mice are completely sterile.
Zhang et al (2024) Nat. Comm. 15:2315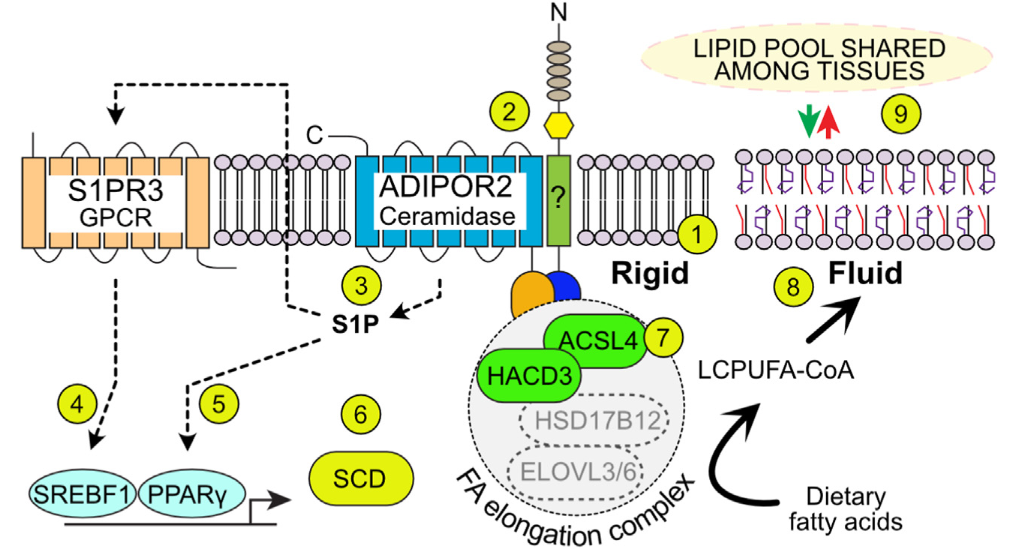
2023: AdipoR2 recruits a fatty acid elongation complex
We found that AdipoR2 and its worm homolog PAQR-2 physically recruit enzymes that elongate fatty acids.
Using immunoprecipitation of tagged proteins, we found that AdipoR2 and PAQR-2 physically interact with several enzymes important for fatty acid metabolism, including FASN and three of the four enzymes required for fatty acid elongation and ACSL4 that can channel long-chain unsaturated fatty acids for incorporation into phospholipids. This provides an additional mechanism by which AdipoR2/PAQR-2 contribute to membrane homeostasis.
Ruiz et al (2023) J. Biol. Chem. 299:104799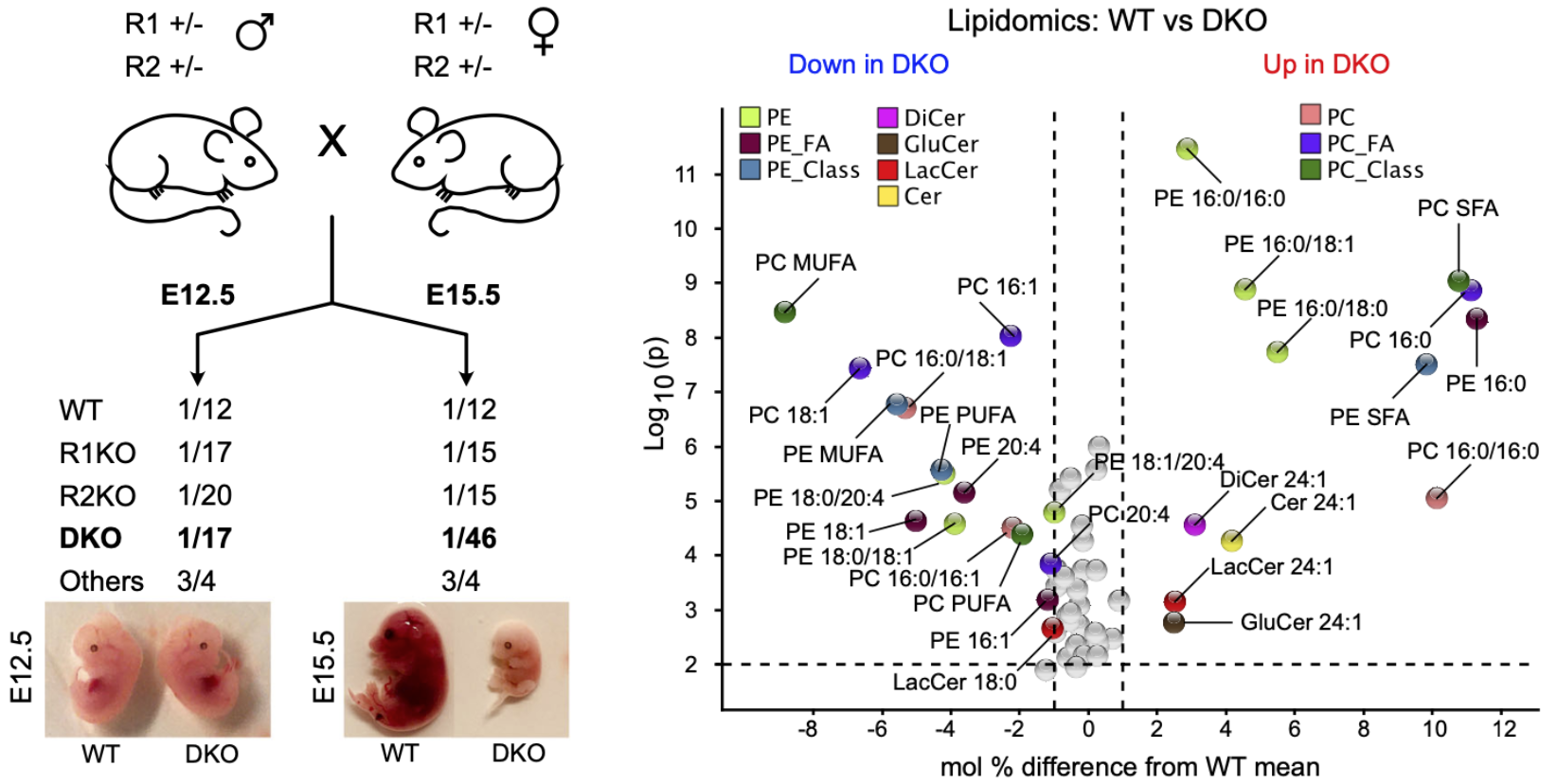
2022: AdipoRs act via sphingosine 1-phosphate
Sphingosine 1-phosphate can abbrogate membrane rigidification in embryonic cells lacking the AdipoRs.
Using mouse embryos, cultivated mammalian cells and the nematode C. elegans, we show that sphingosine 1-phosphate is a signaling molecule acting downstream of the AdipoRs to activate SREBP and PPAR-type transcription factors, leading to increase desaturase activity and improved membrane fluidity.
Ruiz et al (2022) Nat. Comm 13:7162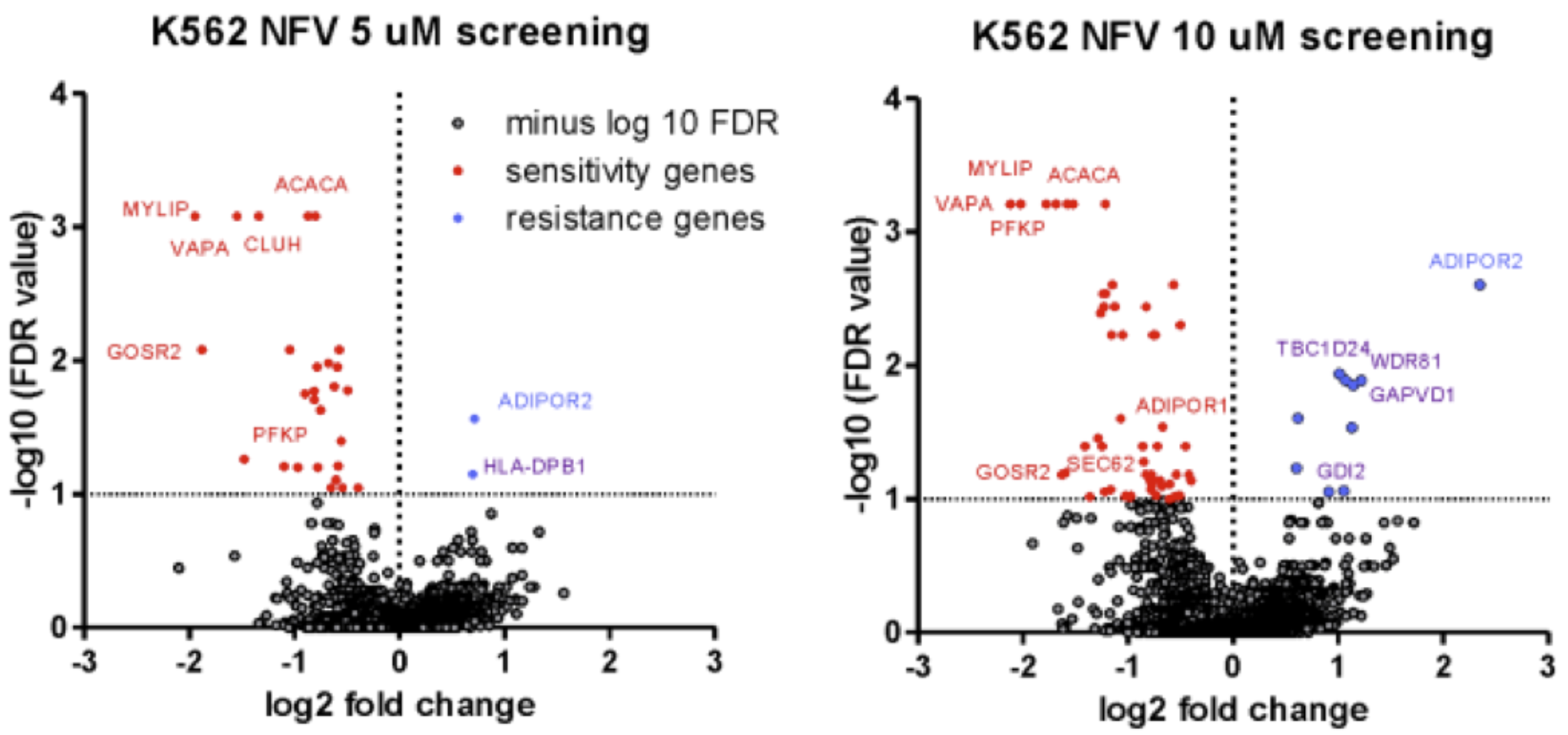
2021: Genome-wide CRISPR/Cas9 screen
A genome-wide CRISPR/Cas9 screen identified mutations in AdipoR2 as the most potent way to achieve Nelfinavir resistance
Nelfinavir is an anti-cancer drug of which the mechanism of action was poorly understood. This CRISPR/Cas9 screen, together with protein-protein interaction studies, cell physiology assays, lipidomics, and membrane fluidity measurements, showed that Nelfinavir acts by interfering with membrane properties. This breakthrough paper was led by our collaborators Lenka and Andrej Besse in the group of Chistoph Driessen in Switzerland.
Besse et al (2021) Cancer Res. 81:4581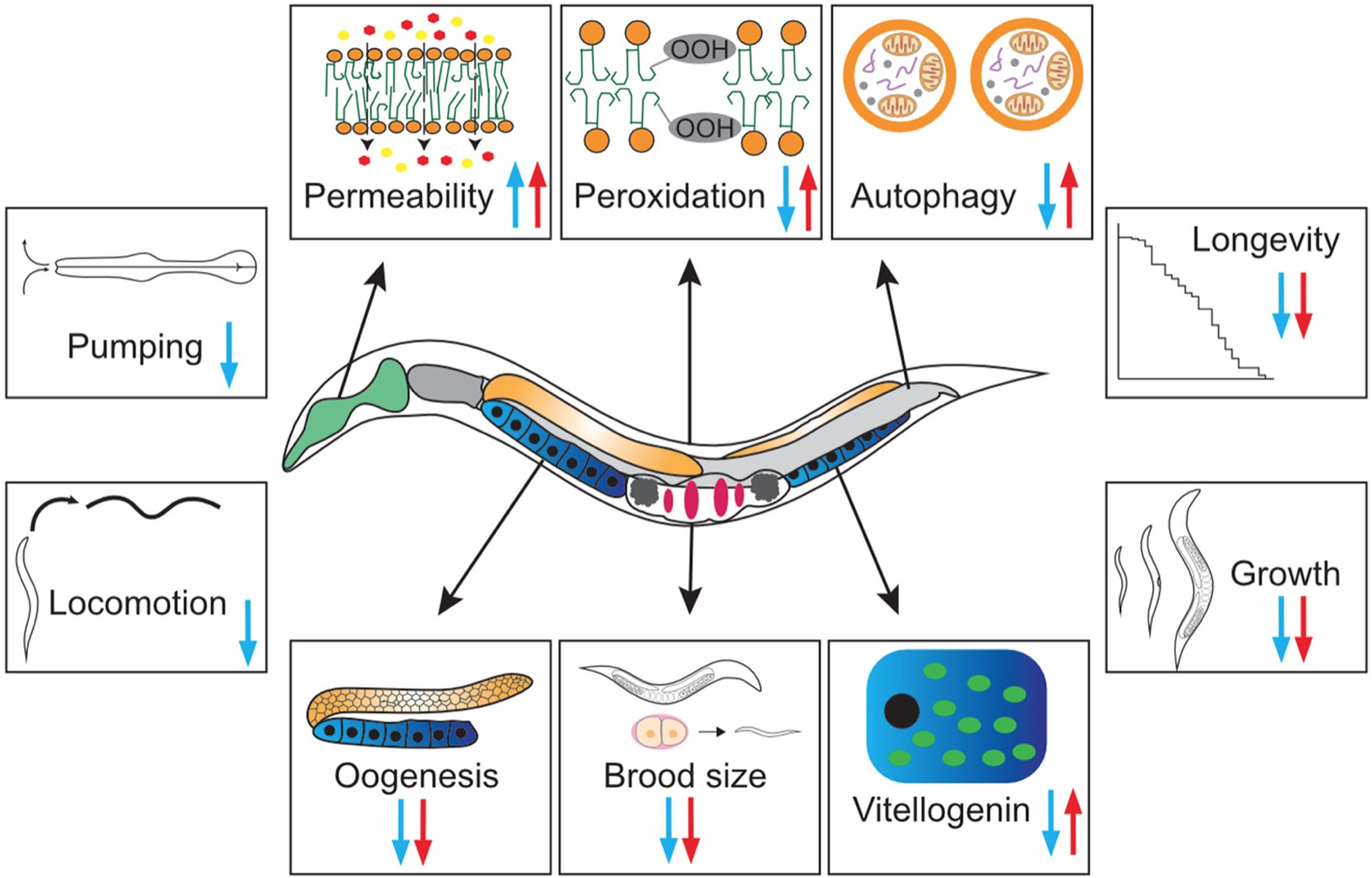
2021: Genetic titration of membrane fluidity
We created a panel of 5 C. elegans strains spanning a wide range of membrane composition/fluidity.
Taking advantage of our genetics expertise concerning alleles that influence membrane composition and fluidity, we created a panel of five strains with varying degree of membrane saturation/fluidity, from extremely rich in saturated fatty acids and therefore rigid, to very rich in polyunsaturated fatty acids and therefore very fluid. The strains with impaired membrane homeostasis showed showed defects in basically every cellular and physiological traits that we examined, though often in contrasting directions when comparing fluid vs rigid strains.
Devkota et al (2021) Genetics 219:iyab093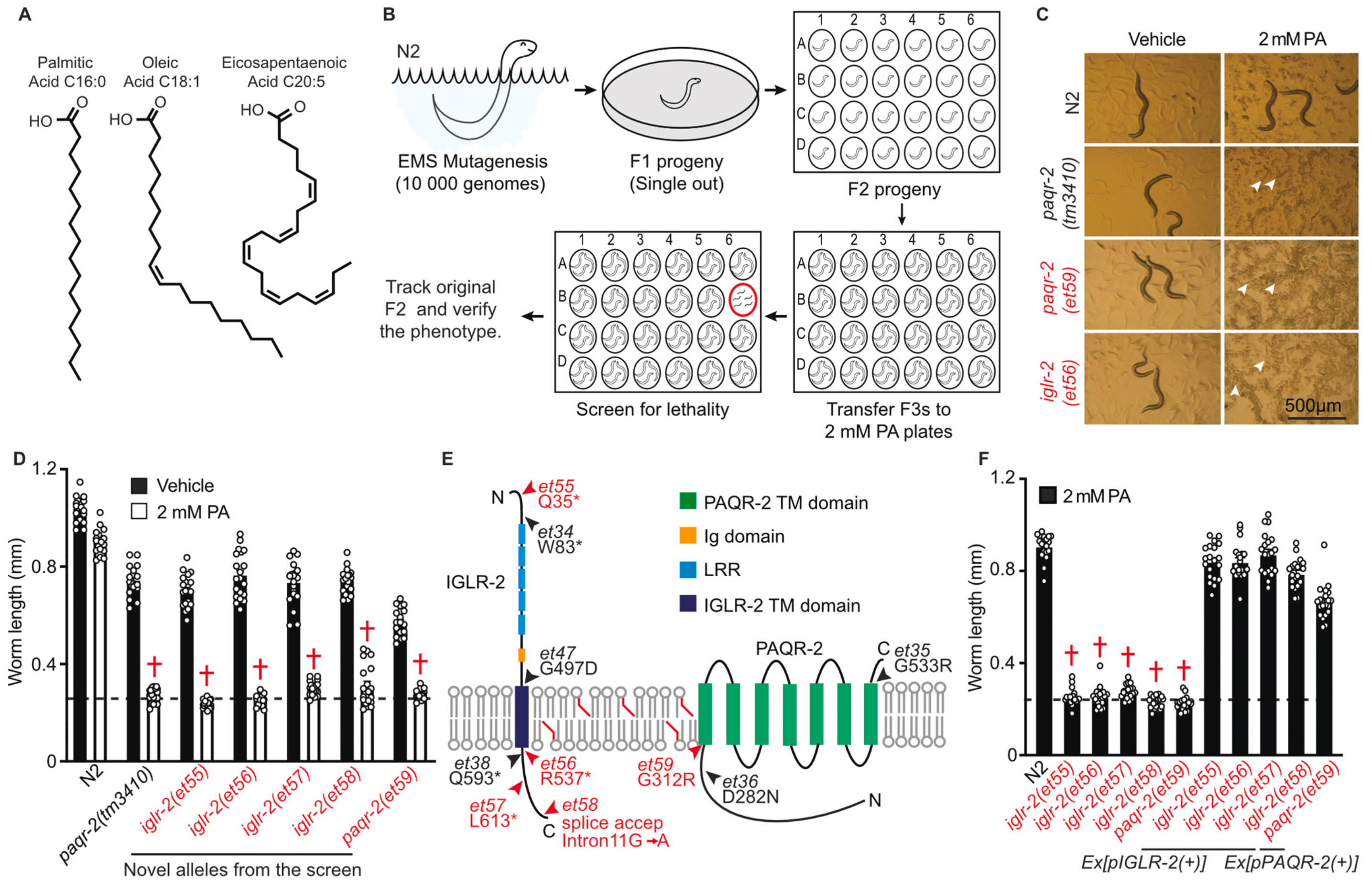
2021: AdipoRs are uniquely essential for tolerating saturated fat
A forward screen in C. elegans picks only PAQR-2 and IGLR-2 as essential for dietary SFA tolerance. A CRISPR/Cas9 human cell mutant lacking AdipoR2 is also SFA intolerant!
Using forward genetics, we found that PAQR-2 and IGLR-2 are the only C. elegans proteins that are specifically essential when the diet contains saturated fat. We also show that the interaction between PAQR-2 and IGLR-2 is induced by membrane rigidification caused by the saturated fatty acids. Separately, we found that mammalian cells lacking AdipoR2 are unable to cope with membrane rigidification and that AdipoR2 is one of the most important genes to allow tolerance to saturated fats. Additionally, thousands of genes are misregulated in the mutant cells challenged with palmitate.
Devkota et al (2021) BBA Mol Cell Biol Lipids 1866:158883Ruiz et al (2021) BBA Mol Cell Biol Lipids 1866:158884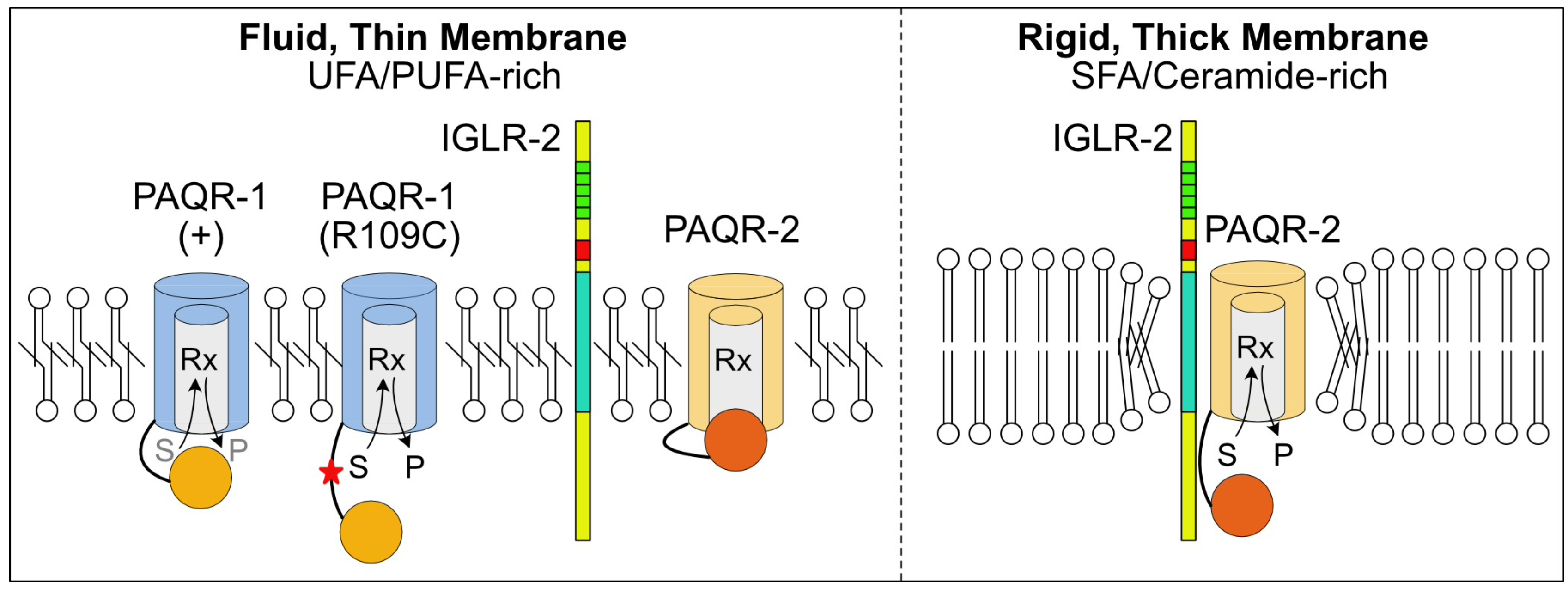
2020: Structure-Function Studies
Domain swapping experiments point to regulatory domains in PAQR proteins
Constructs in which various domains of PAQR-1 and PAQR-2 were swapped allowed us to better understand how these proteins are regulated. In particular, we found that the transmembrane domains of PAQR-2 are responsible for its requirement for IGLR-2. Also, we found that a gain-of-function alelle of PAQR-1, bearing a single point-mutation in the cytoplasmic domain, can functionally replace PAQR-2 and does not require IGLR-2 for its action. This work led to an updated model of PAQR regulation.
Bussayavalasa et al (2020) PLoS Gen 16:e1008975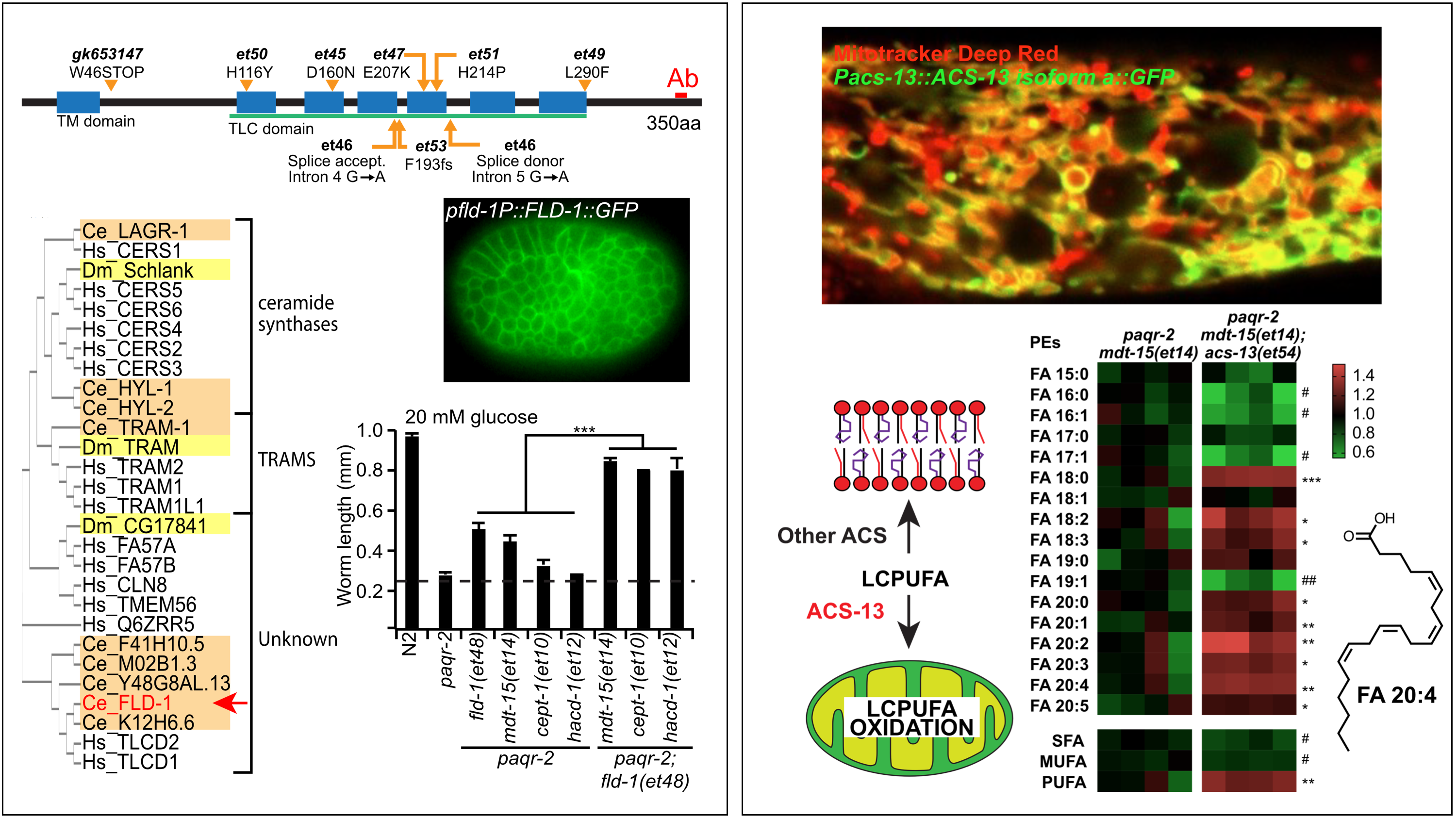
2018/19: PUFA channelling
The AdipoR pathway includes upregulation of desaturases AND channelling of PUFAs into phosphospholipids
Again using forward genetics, we found that there are actually two separate outputs from the PAQR-2/AdipoR pathway when it is activated by membrane rigidification. We knew from earlier that desaturase upragulation is one consequence of PAQR-2/AdipoR signaling. Now, in two separate publications (one about the gene FLD-1/TLCD and the other about the genes acs-13/ACSL1), we show that the pathway also includes a second “branch” that promotes channelling of long-chain polyunsaturated fatty acids (PUFAs) into membrane phospholipids, hence effectively improving membrane fluidity.
Ruiz et al (2019) eLife 8:e47733Ruiz et al (2018) eLife 7:e40686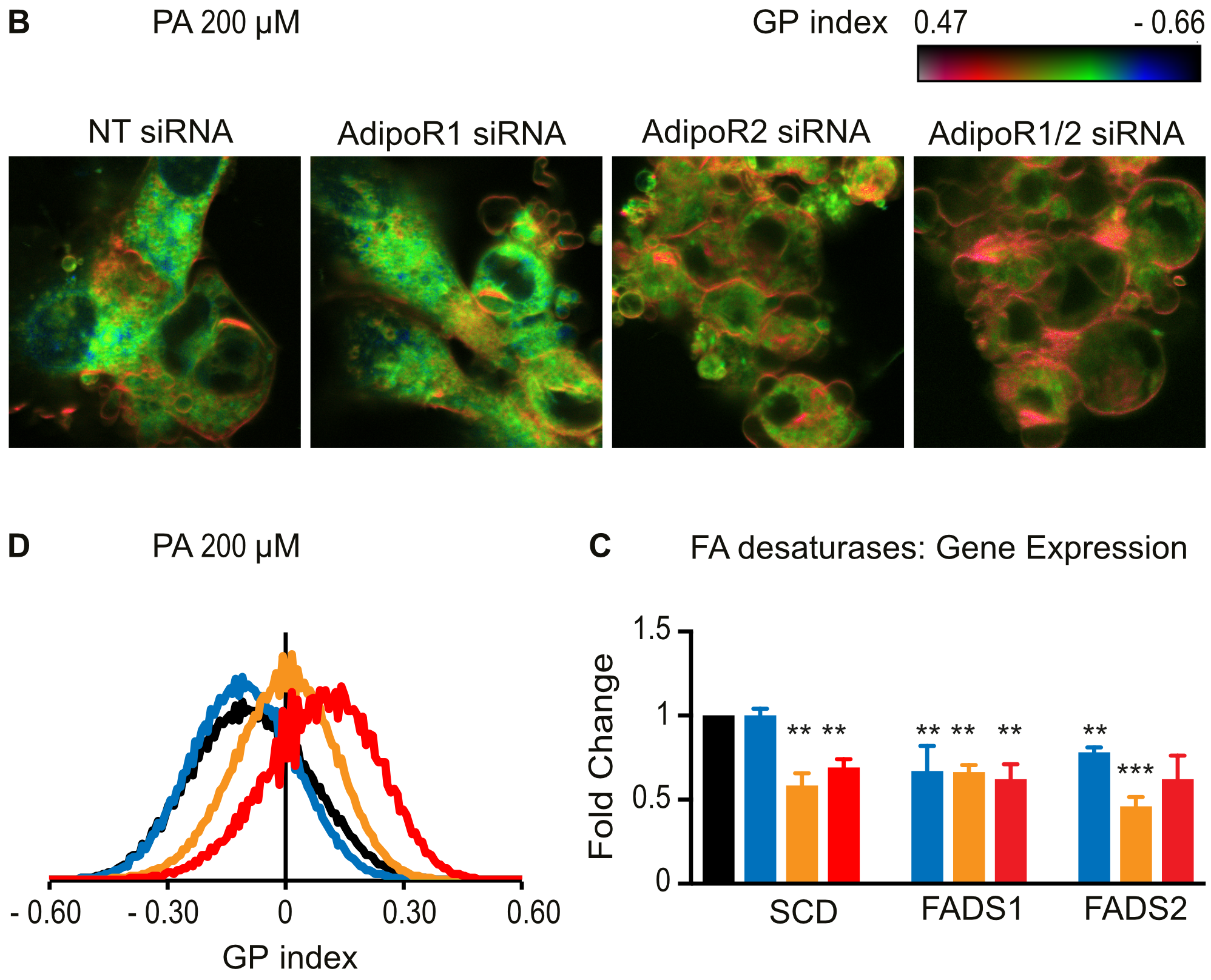
2019: Focus on the human AdipoRs
The human AdipoR proteins act independently of adiponectin to maintain membrane fluidity
We showed that AdipoR1 and AdipoR2 act in all human cells tested (epithelial-derived HEK293, hepatocyte-derive HepG2, astrocyte-derived 1321N1 and primary human umbilical vein endothelial cells) to maintain membrane fluidity and protect against the rigidifying effects of saturated fatty acids. Importantly they can perform this function in the complete absence of adiponectin.
Ruiz et al (2019) J Lipid Res. 60:995-1004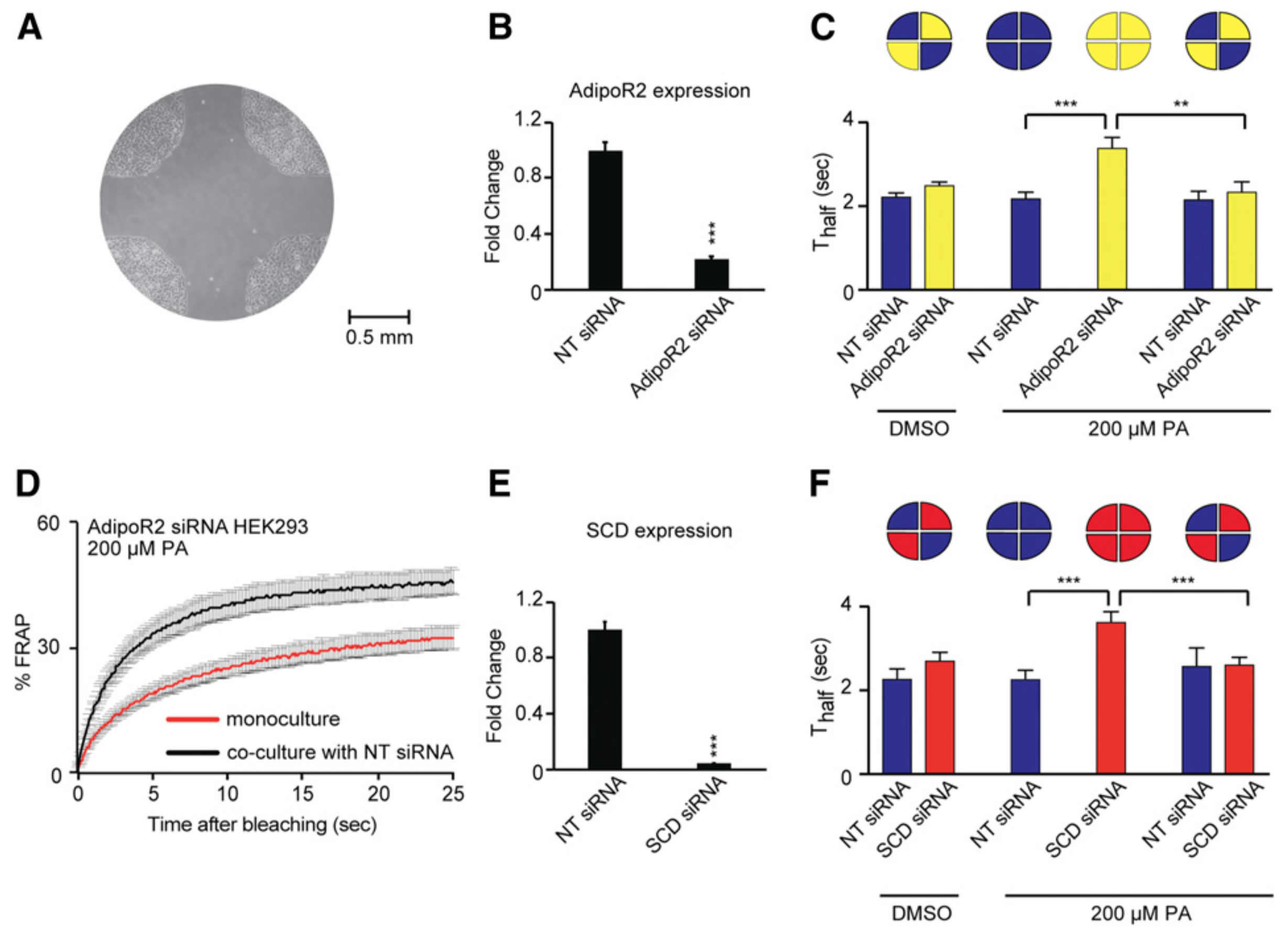
2018: Cell nonautonomous membrane homeostasis
PAQR-2 and AdipoR2 regulate membrane fluidity cell nonautonomously in worms and human cells
We used genetics mosaics and tissue-specific expression in C. elegans to show that PAQR-2 and IGLR-2 expression in some tissues is enough to rescue membrane fluidity in the whole worm. We also used clever partitioned culture dishes to show that this also applies to AdipoR2 in human cells.
This work was a Highlight for the Sept 2018 issue of GENETICS.
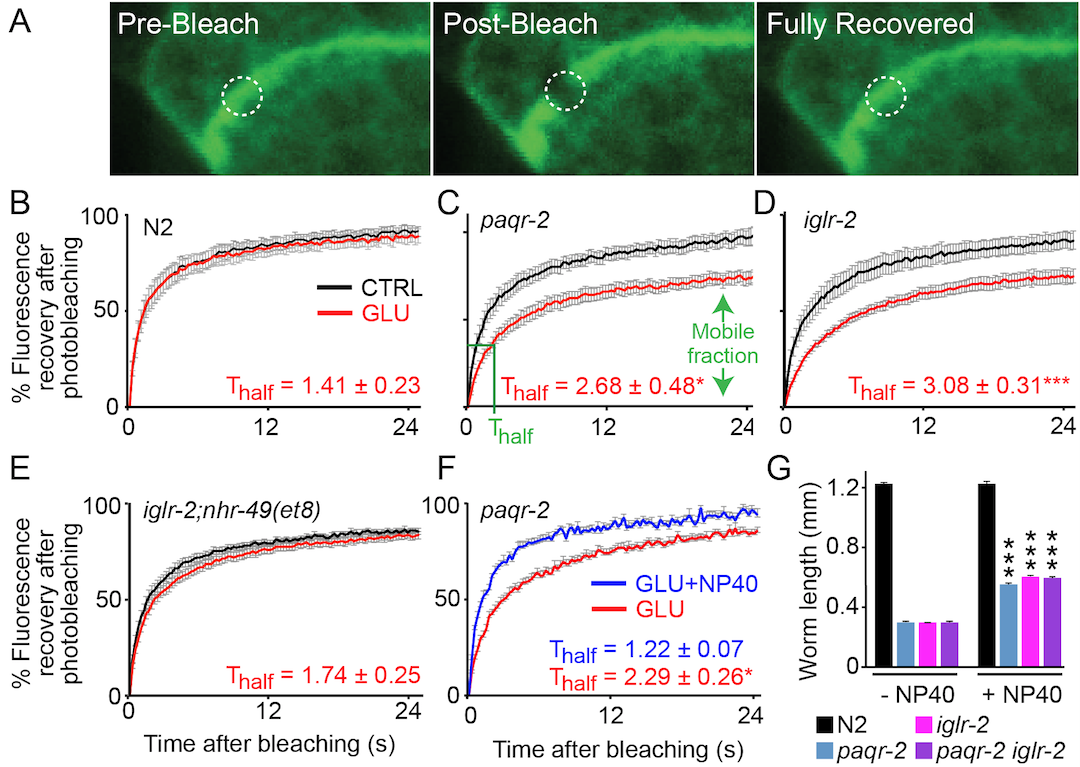
2016: Sugars and Membrane Fluidity
PAQR-2 and IGLR-2 regulate fluidity in C. elegans
We previously showed that PAQR-2, an adiponectin receptor homolog in C. elegans, is essential for cold adaptation in that organism. In 2016 we published our findings that PAQR-2 and IGLR-2 are essential for worms to survive in the presence of glucose: even 10 mM is lethal to the paqr-2 or iglr-2 mutants. We also show that PAQR-2 and IGLR-2 are essential to maintain membrane fluidity in the presence of sugars and that they act by influencing the composition of the cellular membranes, specifically by promoting incorporation of phospholipids with unsaturated fatty acid tails, which improves fluidity.
Svensk et al (2016) PLoS Genetics 12:e1006112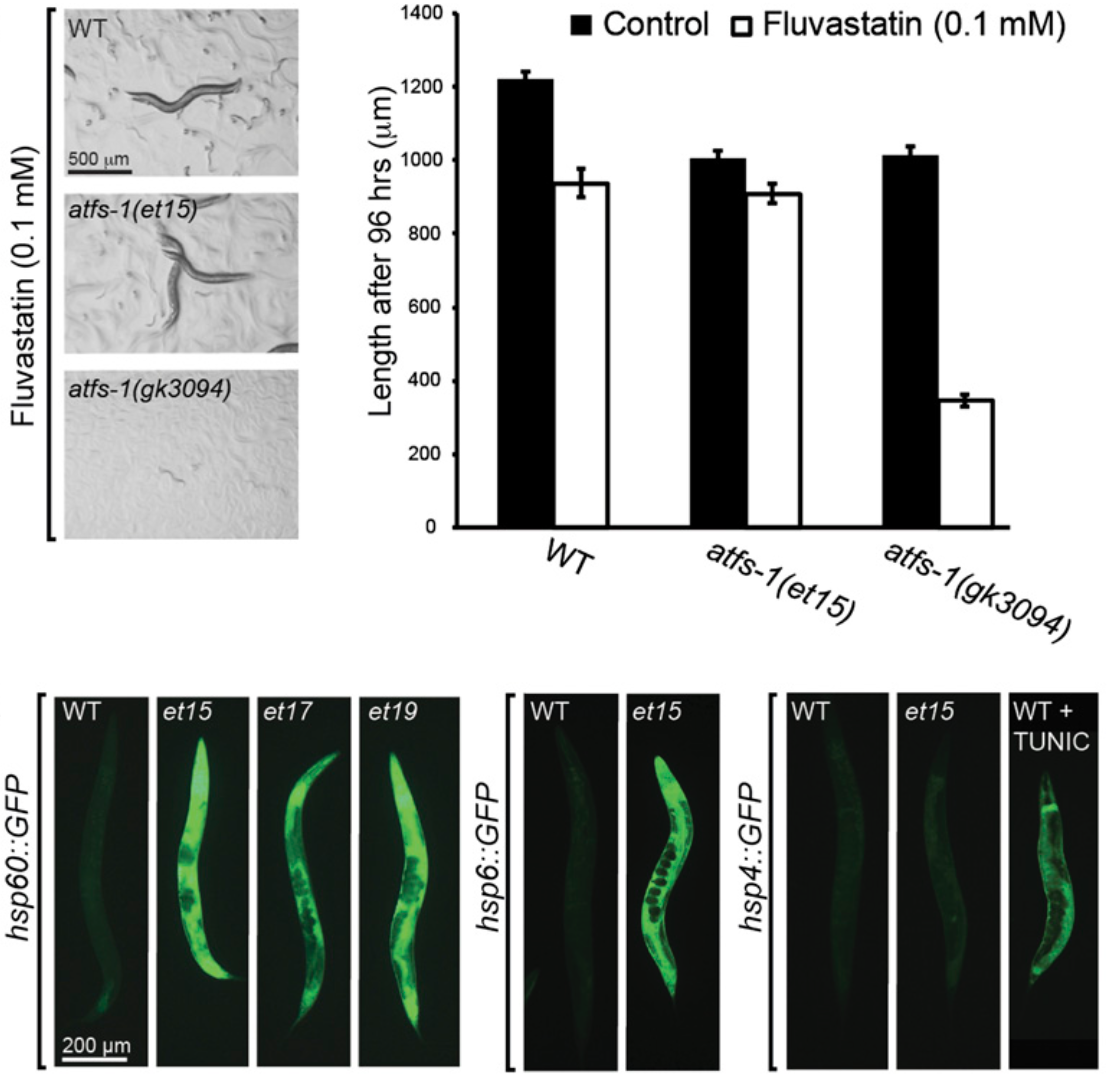
2013: Statins and Mitochondrial Stress
Statin-resistant mutants in C. elegans
We had previously shown that statins are very toxic to the nematode C. elegans in which they impair protein prenylation, a type of lipidation that allows many proteins to associate with membranes. By screening over 150 000 mutagenized haploid C. elegans genomes, we then identified several novel mutants that are resistant to the lethal effects of statins. These mutants all have one thing in common: they have a consitutively activated mitochondrial stress response called the mitorhoncrial UPR. In particular, four of our novel mutants are gain-of-function alleles of ATFS-1, the key positive regulator of mitochondrial UPR. This indicates that one mechanism of statin toxicity that cells have a problem dealing with is by causing mitochondrial stress without triggering the protective response.
Rauthan et al (2013) PNAS 110:5981
2010: Plant-parasitic Nematodes
An eco-friendly control of nematodes in Vietnam
In a project financed by SIDA, we identified the nematode species that destroys a valuable flower crop in the Mekong Delta, and established a method to prevent disease manifestation (immersion of infected bulbs in 56C water for 30 min). The nematode species identification and life cycle characterization was published in 2007, and the hot water treatment and a description of its efficacy compared to pesticides was published in 2010.
Cuc et al (2010) Crop Protection 29:599
2007: Neuronal Development
The fishing-line model of axon guidance
In 2007, we published the cloning of the mnm-2 gene, for which a mutant was isolated in our previous screen for mutants with abnormal morphology of the pharyngeal M2 neuron in C. elegans. mnm-2 encodes a KLF/Sp1/EGF-like zinc finger transcription factor expressed in M3, the sister cell of M2. We discovered that expression of mnm-2 in the M3 cell is essential for that cell’s differentiation and its ability to guide the growth cone of the M2 neuron. We found that the M2 axon develops in two steps. First it is drawn by the M3 being moved away from its M2 sister cell to which the axon tip is initially attached, producing a long straight trajectory (like a fishing line being pulled by a fish). Second, M3 differentiates in an mnm-2-dependent manner and induces a growth cone to form and guide the final trajectories of the distal end of the M2 axon.
Rauthan et al (2007) Dev Biol. 311:185